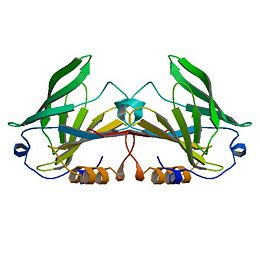
No definite physiological function has been ascribed to β-LG, although several suggestions have been made, the more compelling of which favor a role in molecular transport or, possibly, as some form of modulator (Kontopidis et al., 2002). Most suggestions concerning the function, understandably, have concentrated on either the lactating cell or more usually the neonate, and a transporter role seems reasonable, since many lipocalins are transporters. β-Lactoglobulin binds both fatty acids and retinol, and the structure is similar to the known transporter, plasma RBP. The nature of the ligand transported, apart from being generally hydrophobic, is not clear, however. Fatty acids, rather than retinal, are found as endogenous ligands in milk, but not all species have a β-LG that binds fatty acids (Perez et al., 1989, 1993), and it seems improbable that the true function will vary from species to species. Similarly, retinol is significantly more soluble in the fat phase of milk and thus will probably be transferred from mother to offspring by that route. A signaling or activity-modulator role appears to be less likely, not just because of the paucity of similar roles reported for other lipocalins (Flower et al., 2000), but also because the data supporting these various activities appear rather circumstantial. Further, for such an important role, one might expect the presence of β-LG in the milks of all species, not just some. What does not appear to have been considered in detail until recently (Kontopidis et al., 2002) is that the function is directly related to maternal physiology.
When the species variation of β-LG sequences is examined along with that of the other lipocalins, what emerges is a family tree like that shown in Figure 4. The RBP are clearly distinct from the lactoglobulins, but there is one protein present in the endometrium, now called glycodelin (formerly PP14, inter alia), that is the closest relative to the β-LG-II gene product (Halttunen et al., 2000; Seppälä, 2002). There are also reports of RBP expressed in the endometrium of cow (Thomas et al., 1992; MacKenzie et al., 1997). Notice also that there is ruminant pseudo-gene identified in both cow and goat that is also most nearly related to the sequence of β-LG-II. Might it be that the protein glycodelin reflects the true, original function of β-LG as a protein involved in some aspect of fetal development in all mammals? In many species, the gene has undergone a gene duplication event and is expressed during lactation for nutritional purposes. More recently, some species, like rodents, lagomorphs, and man, have lost the function of one of these genes through formation of a pseudo-gene, resulting in the gene ceasing to be expressed. The biological properties of the milk protein, such as ligand binding and inhibiting harmful bacterial adhesion in the intestine (Ouwehand et al., 1997) that have been identified, are clearly useful to each species but are subsidiary to those of neonatal nutrition. On the other hand, it is known that low levels of β-LG are expressed in the cow throughout the dry period in a manner that is distinct from α-LA and the caseins (Aslam et al., 1994). Whether this is indicative of another more physiological role for β-LG in the changes that occur to the mammary gland during this period remains unclear at present.