G protein-coupled receptor
From Proteopedia
| (121 intermediate revisions not shown.) | |||
| Line 4: | Line 4: | ||
Rhodopsin shares similar membrane topology with the members of the superfamily, specifically family A of the [[G protein-coupled receptor|G protein-coupled receptors]] which include the seven transmembrane helices, an extracellular N-terminus and cytoplasmic C-terminus<ref name="rhodopsin">PMID:15251227</ref>. | Rhodopsin shares similar membrane topology with the members of the superfamily, specifically family A of the [[G protein-coupled receptor|G protein-coupled receptors]] which include the seven transmembrane helices, an extracellular N-terminus and cytoplasmic C-terminus<ref name="rhodopsin">PMID:15251227</ref>. | ||
| + | |||
| + | See also | ||
| + | *[[CAMP-dependent pathway]] | ||
| + | *[[Cytokine receptors]] | ||
| + | *[[Neuropeptides]] | ||
| + | *[[Transmembrane (cell surface) receptors]] | ||
[[Image:7tm labeled.png|right|400px]] | [[Image:7tm labeled.png|right|400px]] | ||
| - | + | {{Clear}} | |
| - | = | + | =List of the G protein-coupled receptors= |
| - | + | ||
| - | + | ||
| - | === | + | ==Family A of GPCRs== |
| - | + | '''Neurotensin receptor''' | |
| + | *[[Neurotensin receptor]] | ||
| + | ===Subfamily A2=== | ||
| + | ====CXC Chemokine receptors==== | ||
| + | * [[CXC chemokine receptor type 4]] | ||
| + | ===Subfamily A4=== | ||
| + | ====Opioid receptors==== | ||
| + | '''Opioid receptor''' (OpR) is a [[G protein-coupled receptor]] with [[opioids]] as ligands<ref>PMID:22204322</ref>. OpR types are classified according to the ligands which bind to them.<br /> | ||
| + | * The '''μ-opioid receptor''' binds morphine. For more details on μ-opioid receptor see<br /> | ||
| + | **[[Mu Opioid Receptor Bound to a Morphinan Antagonist]] | ||
| + | **[[μ Opioid Receptors]] | ||
| + | **[[Mu Opioid Receptor]]. | ||
| + | **[[6dde]] μ-opioid receptor: G protein complex<br /> | ||
| + | * The '''κ-opioid receptor''' binds opium-type ligands. For details see [[Student Project 3 for UMass Chemistry 423 Spring 2015]].<br /> | ||
| + | * The '''δ-opioid receptor''' binds enkephalins. For details see [[Delta opioid receptor]]. | ||
| + | * The '''Nociceptin/orphanin FQ opioid receptor''' binds the heptadecapeptide orphanin<ref>PMID:23395957</ref>. | ||
| + | See also | ||
| + | *[[Opioids]] | ||
| + | *[[Treatments:Opioid drugs]] | ||
| + | *[[Tutorial: The opioid receptor, a molecular switch]] | ||
| + | ===Subfamily A6=== | ||
| + | ====Orexin receptor==== | ||
| + | * [[Orexin and Orexin receptor]] | ||
| + | * [[Belsomra]] and Orexin receptors | ||
| + | * [[Hypocretin and receptors]] | ||
| + | ====[[Oxytocin receptor]]==== | ||
| + | ===Subfamily A10=== | ||
| + | *[[Human Follicle-Stimulating Hormone Complexed with its Receptor]] | ||
| + | ===Subfamily A11=== | ||
| + | ====Human GPR40 (hGPR40), also known as Free Fatty Acid Receptor 1 (FFAR1)==== | ||
| + | * [[GPR40]] | ||
| + | ====Sphingosine 1-phosphate Receptor==== | ||
| + | The sphingosine-1-phosphate receptors are a class of G protein-coupled receptors that are targets of the lipid signalling molecule Sphingosine-1-phosphate (S1P). | ||
| + | * [[3v2w]],[[3v2y]] - human sphingosine 1-phosphate receptor 1 with a bound sphingolipid mimic | ||
| + | See [[User:Harish Srinivas/Sandbox 1]] | ||
| + | ===Subfamily A16=== | ||
| + | ====Rhodopsins==== | ||
| + | The product of light activation, Metarhodopsin II, initiates the visual phototransduction pathway by stimulating the G protein [[Transducin]] (G<sub>t</sub>), resulting in the liberation of its α subunit. This GTP-bound subunit in turn activates cGMP phosphodiesterase. cGMP phosphodiesterase hydrolyzes (breaks down) cGMP, lowering its local concentration so it can no longer activate cGMP-dependent cation channels. Phosphodiesterase 6 is the primary effector of retinal phototransduction. See details in [[User:Rick H. Cote/PDE6]]. | ||
| + | *[[Rhodopsin]] | ||
| + | *[[Rhodopsin Structure and Function]] | ||
| + | *[[Transducin]] | ||
| + | ===Subfamily A17=== | ||
| + | ====5-Hydroxytryptamine (5-HT) receptors (Serotonin receptors)==== | ||
| + | *[[5-hydroxytryptamine receptor|Serotonin receptors, main page]] | ||
| + | *[[5-hydroxytryptamine receptor 3D structures|3D structures of Serotonin receptors]] | ||
| + | ====Adrenergic receptors==== | ||
| + | * [[Adrenergic receptor|Adrenergic receptors in general]] | ||
| + | =====β1 adrenergic receptor===== | ||
| + | *3D structures in [[Adrenergic receptor]]. | ||
| + | *[[UMass Chem 423 Student Projects 2011-1#Beta-1 Adrenergic GPCR|Beta-1 Adrenergic receptor]] | ||
| + | *G<sub>s</sub>: [[adenylate cyclase]] activated, cAMP up. | ||
| + | *β1-adrenergic agonists: | ||
| + | **Dobutamine, see [[UMass Chem 423 Student Projects 2011-1#Beta-1 Adrenergic GPCR|Beta-1 Adrenergic receptor]], [[2y00]], [[2y01]], [[6h7l]]. | ||
| + | **Isoprenaline, see [[UMass Chem 423 Student Projects 2011-1#Beta-1 Adrenergic GPCR|Beta-1 Adrenergic receptor]], [[2y03]]. | ||
| + | **Noradrenaline | ||
| + | **Carmoterol, see [[2y02]]. | ||
| + | **[[Salbutamol]] (Albuterol in USA), [[2y04]]. | ||
| + | *Beta blockers: | ||
| + | **Metoprolol | ||
| + | **Atenolol | ||
| + | **Bisoprolol | ||
| + | **[[Propranolol]] | ||
| + | **[[Timolol]] | ||
| + | **Nebivolol | ||
| + | **Vortioxetine | ||
| + | =====β2 adrenergic receptor===== | ||
| + | {{Template:GPCR3sn6}} | ||
| + | {{Clear}} | ||
| + | * The human β2 adrenergic receptor bound to a G-protein ([[3sn6]]) is featured in a scene above, and additional structures are on the [[Adrenergic receptor|Adrenergic receptor page]]. | ||
| + | *[[Beta-2 Adrenergic Receptor|Article Beta-2 Adrenergic Receptor by Wayne Decatur, David Canner, Dotan Shaniv, Joel L. Sussman, Michal Harel]] | ||
| + | *[[Beta-2 adrenergic receptor|Article Beta-2 adrenergic receptor by Joel L. Sussman, Tala Curry, Michal Harel, Jaime Prilusky]] | ||
| + | *[[Group:SMART:A Physical Model of the beta-Adrenergic Receptor]] | ||
| + | *G<sub>s</sub>: adenylate cyclase activated, cAMP up. For G<sub>s</sub> see [[Beta2 adrenergic receptor-Gs protein complex updated]]. | ||
| + | β2-adrenergic agonists: | ||
| + | **[[Salbutamol]] (Albuterol in USA) | ||
| + | **Bitolterol mesylate | ||
| + | **[[Formoterol]] | ||
| + | **Isoprenaline | ||
| + | **Levalbuterol | ||
| + | **Metaproterenol | ||
| + | **[[Salmeterol]] | ||
| + | **Terbutaline | ||
| + | **Ritodrine | ||
| + | *Beta blockers: | ||
| + | **Butoxamine | ||
| + | **[[Timolol]] | ||
| + | **[[Propranolol]] | ||
| + | **ICI-118,551 | ||
| + | **Paroxetine | ||
| + | ====Dopamine Receptor==== | ||
| + | There are five subtype dopamine receptors, D1, D2, D3, D4, and D5. The D3 receptor is a part of the D2-like family.<ref>PMID:15148138</ref> The D2-like family receptors are coupled to the G protein Giα, which directly inhibits the formation of cAMP by inhibiting the enzyme adenylyl cyclase. | ||
| + | *[[Dopamine receptor|Dopamine receptors 1 page]] | ||
| + | *[[Dopamine Receptors|Dopamine receptors 2 page]] | ||
| + | ===Subfamily A18=== | ||
| + | ====Histamine receptors==== | ||
| + | The H1 receptor is a histamine receptor belonging to the family of rhodopsin-like G-protein-coupled receptors. The H1 receptor is linked to an intracellular G-protein (G<sub>q</sub>) that activates [[phospholipase C]] (see [[PLC beta 3 Gq|Unique bidirectional interactions of Phospholipase C beta 3 with G alpha Q]] and the inositol triphosphate (IP3) signalling pathway. When a ligand binds to a G protein-coupled receptorthat is coupled to a G<sub>q</sub> heterotrimeric G protein, the α-subunit of G<sub>q</sub> can bind to and induce activity in the PLC isozyme PLC-β, which results in the cleavage of PIP2 into IP3 and DAG. | ||
| + | *[[Histamine H1 receptor]] | ||
| + | * [[3rze]] - human histamine H1 receptor with an antagonist doxepin. | ||
| + | ====Adenosine A2A receptor==== | ||
| + | G<sub>s</sub> → cAMP up | ||
| + | *[[Adenosine A2A receptor]] | ||
| + | *[[Caffeine|Effect of Caffeine (Trimethylxanthine) on Human A2A Receptor]] | ||
| + | *[[Adenosine A2A receptor 3D structures]] | ||
| + | Agonists: | ||
| + | *N6-3-methoxyl-4-hydroxybenzyl adenine riboside (B2) | ||
| + | *ATL-146e | ||
| + | *CGS-21680 | ||
| + | *Regadenoson | ||
| + | *Adenosine | ||
| + | Antagonists: | ||
| + | *Caffeine | ||
| + | *aminophylline | ||
| + | *theophylline | ||
| + | *istradefylline | ||
| + | *SCH-58261 | ||
| + | *SCH-442,416 | ||
| + | *ZM-241,385 | ||
| - | + | ====Muscarinic acetylcholine receptors==== | |
| - | + | M1, M3, M5 receptors are coupled with G<sub>q</sub> proteins, while M2 and M4 receptors are coupled with G<sub>i/o</sub> proteins. | |
| - | + | *[[Muscarinic acetylcholine receptor]] | |
| - | == | + | ==Family B of GPCRs== |
| - | + | These receptors activate adenylyl cyclase and the phosphatidyl-inositol-calcium pathway. | |
| + | The glucagon receptor is a 62 kDa protein that is activated by glucagon and is a member of the class B G-protein coupled family of receptors, coupled to G alpha i, Gs and to a lesser extent G alpha q. Stimulation of the receptor results in the activation of [[adenylate cyclase]] and [[phospholipase C]] and in increased levels of the secondary messengers intracellular cAMP and calcium. | ||
| - | === | + | ===Subfamily B1=== |
| - | + | ====Glucose-dependent Insulinotropic Polypeptide Receptor==== | |
| - | * [[ | + | * [[Glucose-dependent Insulinotropic Polypeptide Receptor]] |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | === | + | ====Glucagon receptor==== |
| - | * [[ | + | * [[Glucagon receptor]] |
| - | === | + | ====Glucagon-like peptide 1 receptor==== |
| - | * [[ | + | * [[Glucagon-like peptide 1 receptor]] |
| - | === | + | ==Family C of GPCRs, Metabotropic glutamate receptors== |
| - | + | ||
| - | + | Metabotropic glutamate receptors are [[Glutamate Receptors|glutamate receptors]] that activate ion channels indirectly through a signaling cascade involving G proteins<ref>PMID:20716669</ref>. Glutamate receptors are classified into 3 groups based on their homology, mechanism and pharmacological properties. | |
| - | + | *[[Metabotropic glutamate receptor|Metabotropic Glutamate Receptors]] | |
| + | *[[Ligand Binding N-Terminal of Metabotropic Glutamate Receptors]] | ||
| + | *[[Metabotropic glutamate receptor 5]] | ||
| - | == | + | ==Metabotropic GABA receptors (GABAB)== |
| - | * [[ | + | GABAB receptors (GABABR) are G-protein coupled receptors for gamma-aminobutyric acid (GABA), therefore making them metabotropic receptors, that are linked via G-proteins to potassium channels. GABAB receptors also reduces the activity of adenylyl cyclase and Ca2+ channels by using G-proteins with Gi/G0 α subunits. |
| + | *[[GABA receptor]] | ||
| + | *[[User:Rana Saad/The human GABAb receptor]] | ||
| - | + | =Nobel Prize Related to the Structures= | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
[http://www.nobelprize.org/nobel_prizes/chemistry/laureates/2012/ Robert J. Lefkowitz and Brian K. Kobilka share the 2012 Nobel Prize in Chemistry] for work on GPCRs that includes solving the first structures of a ligand-activated GPCR ([[2r4r]], [[2r4s]], & [[2rh1]] in 2007)<ref>PMID: 17962520</ref><ref>PMID: 17962519</ref><ref>PMID: 18033872</ref> and the first activated GPCR in complex with its G protein ([[3sn6]] in 2011)<ref>PMID: 21956322</ref><ref>PMID: 21772288</ref><ref> PMID: 21956331</ref><ref>PMID: 21956322</ref>. A detailed description of the laureates' body of work on this class of receptors with images is [http://www.nobelprize.org/nobel_prizes/chemistry/laureates/2012/popular-chemistryprize2012.pdf here]. | [http://www.nobelprize.org/nobel_prizes/chemistry/laureates/2012/ Robert J. Lefkowitz and Brian K. Kobilka share the 2012 Nobel Prize in Chemistry] for work on GPCRs that includes solving the first structures of a ligand-activated GPCR ([[2r4r]], [[2r4s]], & [[2rh1]] in 2007)<ref>PMID: 17962520</ref><ref>PMID: 17962519</ref><ref>PMID: 18033872</ref> and the first activated GPCR in complex with its G protein ([[3sn6]] in 2011)<ref>PMID: 21956322</ref><ref>PMID: 21772288</ref><ref> PMID: 21956331</ref><ref>PMID: 21956322</ref>. A detailed description of the laureates' body of work on this class of receptors with images is [http://www.nobelprize.org/nobel_prizes/chemistry/laureates/2012/popular-chemistryprize2012.pdf here]. | ||
| - | + | =References and Notes= | |
| - | + | ||
<references/> | <references/> | ||
| - | + | =See Also= | |
* [[Nobel Prizes for 3D Molecular Structure]] | * [[Nobel Prizes for 3D Molecular Structure]] | ||
* [[Highest impact structures]] of all time | * [[Highest impact structures]] of all time | ||
| Line 119: | Line 196: | ||
*[[Membrane proteins]] | *[[Membrane proteins]] | ||
*[[Hormone]] | *[[Hormone]] | ||
| + | *[[Ligand Binding N-Terminal of Metabotropic Glutamate Receptors]] | ||
| + | *[[Receptor]]. | ||
| - | + | =Additional Literature= | |
| - | <ref group="xtra">PMID: | + | <ref group="xtra">PMID: 28482214</ref><references group="xtra"/> |
==External Resources== | ==External Resources== | ||
| Line 138: | Line 217: | ||
* [http://nava.liacs.nl/ GPCR Natural Variants Database (NaVa)] | * [http://nava.liacs.nl/ GPCR Natural Variants Database (NaVa)] | ||
* [http://athina.biol.uoa.gr/bioinformatics/PRED-GPCR/ The PRED-GPCR server] for GPCR recognition and family classification. | * [http://athina.biol.uoa.gr/bioinformatics/PRED-GPCR/ The PRED-GPCR server] for GPCR recognition and family classification. | ||
| - | + | * [http://zhanglab.ccmb.med.umich.edu/GLASS GLASS database: a comprehensive database for experimentally validated GPCR-ligand associations] | |
| + | *[http://open.gpcr-modsim.org/ GPCR-ModSim] is a webserver for computational modeling and simulation of G-Protein Coupled Receptors (GPCRs). Models from sequence and also lets you place the models in a phospholipid bilayer and model them using Molecular Dynamics. | ||
[[Category:Topic Page]] | [[Category:Topic Page]] | ||
Current revision
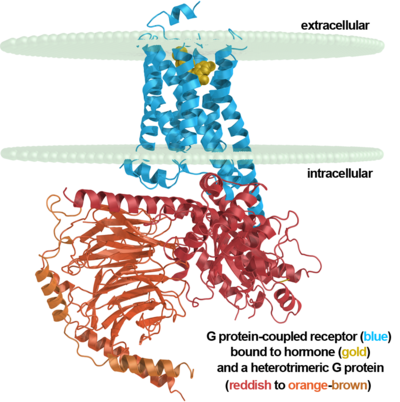
G protein-coupled receptors, often abbreviated GPCRs, are an abundant superfamily of proteins also known as seven-transmembrane domain receptors, 7TM receptors, 7 pass transmembrane receptors, heptahelical receptors, serpentine receptor, and G protein-linked receptors (GPLRs). G protein-coupled receptors are cell surface signalling proteins involved in many physiological functions and in multiple diseases. They are also the target of the majority of all modern medicinal drugs[1][2]. The extracellular side is generally where the ligand enters for binding. On the intracellular side they interact with G proteins involved in signaling induced by the binding of the ligand.
Illustrating their importance and the largesse of the superfamily, there are roughly 800 known members of the superfamily in the human genome alone — estimated to be 4% of human protein-coding genes. Members are further subclassified into one of five families of GPCRs[3].
Rhodopsin shares similar membrane topology with the members of the superfamily, specifically family A of the G protein-coupled receptors which include the seven transmembrane helices, an extracellular N-terminus and cytoplasmic C-terminus[4].
See also
Contents
|
List of the G protein-coupled receptors
Family A of GPCRs
Neurotensin receptor
Subfamily A2
CXC Chemokine receptors
Subfamily A4
Opioid receptors
Opioid receptor (OpR) is a G protein-coupled receptor with opioids as ligands[5]. OpR types are classified according to the ligands which bind to them.
- The μ-opioid receptor binds morphine. For more details on μ-opioid receptor see
- Mu Opioid Receptor Bound to a Morphinan Antagonist
- μ Opioid Receptors
- Mu Opioid Receptor.
- 6dde μ-opioid receptor: G protein complex
- The κ-opioid receptor binds opium-type ligands. For details see Student Project 3 for UMass Chemistry 423 Spring 2015.
- The δ-opioid receptor binds enkephalins. For details see Delta opioid receptor.
- The Nociceptin/orphanin FQ opioid receptor binds the heptadecapeptide orphanin[6].
See also
Subfamily A6
Orexin receptor
- Orexin and Orexin receptor
- Belsomra and Orexin receptors
- Hypocretin and receptors
Oxytocin receptor
Subfamily A10
Subfamily A11
Human GPR40 (hGPR40), also known as Free Fatty Acid Receptor 1 (FFAR1)
Sphingosine 1-phosphate Receptor
The sphingosine-1-phosphate receptors are a class of G protein-coupled receptors that are targets of the lipid signalling molecule Sphingosine-1-phosphate (S1P).
See User:Harish Srinivas/Sandbox 1
Subfamily A16
Rhodopsins
The product of light activation, Metarhodopsin II, initiates the visual phototransduction pathway by stimulating the G protein Transducin (Gt), resulting in the liberation of its α subunit. This GTP-bound subunit in turn activates cGMP phosphodiesterase. cGMP phosphodiesterase hydrolyzes (breaks down) cGMP, lowering its local concentration so it can no longer activate cGMP-dependent cation channels. Phosphodiesterase 6 is the primary effector of retinal phototransduction. See details in User:Rick H. Cote/PDE6.
Subfamily A17
5-Hydroxytryptamine (5-HT) receptors (Serotonin receptors)
Adrenergic receptors
β1 adrenergic receptor
- 3D structures in Adrenergic receptor.
- Beta-1 Adrenergic receptor
- Gs: adenylate cyclase activated, cAMP up.
- β1-adrenergic agonists:
- Dobutamine, see Beta-1 Adrenergic receptor, 2y00, 2y01, 6h7l.
- Isoprenaline, see Beta-1 Adrenergic receptor, 2y03.
- Noradrenaline
- Carmoterol, see 2y02.
- Salbutamol (Albuterol in USA), 2y04.
- Beta blockers:
- Metoprolol
- Atenolol
- Bisoprolol
- Propranolol
- Timolol
- Nebivolol
- Vortioxetine
β2 adrenergic receptor
| |||||||||||
| |||||||||||
- The human β2 adrenergic receptor bound to a G-protein (3sn6) is featured in a scene above, and additional structures are on the Adrenergic receptor page.
- Article Beta-2 Adrenergic Receptor by Wayne Decatur, David Canner, Dotan Shaniv, Joel L. Sussman, Michal Harel
- Article Beta-2 adrenergic receptor by Joel L. Sussman, Tala Curry, Michal Harel, Jaime Prilusky
- Group:SMART:A Physical Model of the beta-Adrenergic Receptor
- Gs: adenylate cyclase activated, cAMP up. For Gs see Beta2 adrenergic receptor-Gs protein complex updated.
β2-adrenergic agonists:
- Salbutamol (Albuterol in USA)
- Bitolterol mesylate
- Formoterol
- Isoprenaline
- Levalbuterol
- Metaproterenol
- Salmeterol
- Terbutaline
- Ritodrine
- Beta blockers:
- Butoxamine
- Timolol
- Propranolol
- ICI-118,551
- Paroxetine
Dopamine Receptor
There are five subtype dopamine receptors, D1, D2, D3, D4, and D5. The D3 receptor is a part of the D2-like family.[7] The D2-like family receptors are coupled to the G protein Giα, which directly inhibits the formation of cAMP by inhibiting the enzyme adenylyl cyclase.
Subfamily A18
Histamine receptors
The H1 receptor is a histamine receptor belonging to the family of rhodopsin-like G-protein-coupled receptors. The H1 receptor is linked to an intracellular G-protein (Gq) that activates phospholipase C (see Unique bidirectional interactions of Phospholipase C beta 3 with G alpha Q and the inositol triphosphate (IP3) signalling pathway. When a ligand binds to a G protein-coupled receptorthat is coupled to a Gq heterotrimeric G protein, the α-subunit of Gq can bind to and induce activity in the PLC isozyme PLC-β, which results in the cleavage of PIP2 into IP3 and DAG.
- Histamine H1 receptor
- 3rze - human histamine H1 receptor with an antagonist doxepin.
Adenosine A2A receptor
Gs → cAMP up
- Adenosine A2A receptor
- Effect of Caffeine (Trimethylxanthine) on Human A2A Receptor
- Adenosine A2A receptor 3D structures
Agonists:
- N6-3-methoxyl-4-hydroxybenzyl adenine riboside (B2)
- ATL-146e
- CGS-21680
- Regadenoson
- Adenosine
Antagonists:
- Caffeine
- aminophylline
- theophylline
- istradefylline
- SCH-58261
- SCH-442,416
- ZM-241,385
Muscarinic acetylcholine receptors
M1, M3, M5 receptors are coupled with Gq proteins, while M2 and M4 receptors are coupled with Gi/o proteins.
Family B of GPCRs
These receptors activate adenylyl cyclase and the phosphatidyl-inositol-calcium pathway. The glucagon receptor is a 62 kDa protein that is activated by glucagon and is a member of the class B G-protein coupled family of receptors, coupled to G alpha i, Gs and to a lesser extent G alpha q. Stimulation of the receptor results in the activation of adenylate cyclase and phospholipase C and in increased levels of the secondary messengers intracellular cAMP and calcium.
Subfamily B1
Glucose-dependent Insulinotropic Polypeptide Receptor
Glucagon receptor
Glucagon-like peptide 1 receptor
Family C of GPCRs, Metabotropic glutamate receptors
Metabotropic glutamate receptors are glutamate receptors that activate ion channels indirectly through a signaling cascade involving G proteins[8]. Glutamate receptors are classified into 3 groups based on their homology, mechanism and pharmacological properties.
- Metabotropic Glutamate Receptors
- Ligand Binding N-Terminal of Metabotropic Glutamate Receptors
- Metabotropic glutamate receptor 5
Metabotropic GABA receptors (GABAB)
GABAB receptors (GABABR) are G-protein coupled receptors for gamma-aminobutyric acid (GABA), therefore making them metabotropic receptors, that are linked via G-proteins to potassium channels. GABAB receptors also reduces the activity of adenylyl cyclase and Ca2+ channels by using G-proteins with Gi/G0 α subunits.
Nobel Prize Related to the Structures
Robert J. Lefkowitz and Brian K. Kobilka share the 2012 Nobel Prize in Chemistry for work on GPCRs that includes solving the first structures of a ligand-activated GPCR (2r4r, 2r4s, & 2rh1 in 2007)[9][10][11] and the first activated GPCR in complex with its G protein (3sn6 in 2011)[12][13][14][15]. A detailed description of the laureates' body of work on this class of receptors with images is here.
References and Notes
- ↑ Overington JP, Al-Lazikani B, Hopkins AL. How many drug targets are there? Nat Rev Drug Discov. 2006 Dec;5(12):993-6. PMID:17139284 doi:10.1038/nrd2199
- ↑ Peeters MC, van Westen GJ, Li Q, IJzerman AP. Importance of the extracellular loops in G protein-coupled receptors for ligand recognition and receptor activation. Trends Pharmacol Sci. 2011 Jan;32(1):35-42. PMID:21075459 doi:10.1016/j.tips.2010.10.001
- ↑ Millar RP, Newton CL. The year in G protein-coupled receptor research. Mol Endocrinol. 2010 Jan;24(1):261-74. Epub 2009 Dec 17. PMID:20019124 doi:10.1210/me.2009-0473
- ↑ Kristiansen K. Molecular mechanisms of ligand binding, signaling, and regulation within the superfamily of G-protein-coupled receptors: molecular modeling and mutagenesis approaches to receptor structure and function. Pharmacol Ther. 2004 Jul;103(1):21-80. PMID:15251227 doi:10.1016/j.pharmthera.2004.05.002
- ↑ Feng Y, He X, Yang Y, Chao D, Lazarus LH, Xia Y. Current research on opioid receptor function. Curr Drug Targets. 2012 Feb;13(2):230-46. PMID:22204322
- ↑ Donica CL, Awwad HO, Thakker DR, Standifer KM. Cellular mechanisms of nociceptin/orphanin FQ (N/OFQ) peptide (NOP) receptor regulation and heterologous regulation by N/OFQ. Mol Pharmacol. 2013 May;83(5):907-18. doi: 10.1124/mol.112.084632. Epub 2013 Feb , 8. PMID:23395957 doi:http://dx.doi.org/10.1124/mol.112.084632
- ↑ Girault JA, Greengard P. The neurobiology of dopamine signaling. Arch Neurol. 2004 May;61(5):641-4. PMID:15148138 doi:10.1001/archneur.61.5.641
- ↑ Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan H, Myers SJ, Dingledine R. Glutamate receptor ion channels: structure, regulation, and function. Pharmacol Rev. 2010 Sep;62(3):405-96. doi: 10.1124/pr.109.002451. PMID:20716669 doi:http://dx.doi.org/10.1124/pr.109.002451
- ↑ Cherezov V, Rosenbaum DM, Hanson MA, Rasmussen SG, Thian FS, Kobilka TS, Choi HJ, Kuhn P, Weis WI, Kobilka BK, Stevens RC. High-resolution crystal structure of an engineered human beta2-adrenergic G protein-coupled receptor. Science. 2007 Nov 23;318(5854):1258-65. Epub 2007 Oct 25. PMID:17962520
- ↑ Rosenbaum DM, Cherezov V, Hanson MA, Rasmussen SG, Thian FS, Kobilka TS, Choi HJ, Yao XJ, Weis WI, Stevens RC, Kobilka BK. GPCR engineering yields high-resolution structural insights into beta2-adrenergic receptor function. Science. 2007 Nov 23;318(5854):1266-73. Epub 2007 Oct 25. PMID:17962519
- ↑ Ranganathan R. Biochemistry. Signaling across the cell membrane. Science. 2007 Nov 23;318(5854):1253-4. PMID:18033872 doi:10.1126/science.1151656
- ↑ Schwartz TW, Sakmar TP. Structural biology: snapshot of a signalling complex. Nature. 2011 Sep 28;477(7366):540-1. doi: 10.1038/477540a. PMID:21956322 doi:10.1038/477540a
- ↑ Rasmussen SG, DeVree BT, Zou Y, Kruse AC, Chung KY, Kobilka TS, Thian FS, Chae PS, Pardon E, Calinski D, Mathiesen JM, Shah ST, Lyons JA, Caffrey M, Gellman SH, Steyaert J, Skiniotis G, Weis WI, Sunahara RK, Kobilka BK. Crystal structure of the beta2 adrenergic receptor-Gs protein complex. Nature. 2011 Jul 19;477(7366):549-55. doi: 10.1038/nature10361. PMID:21772288 doi:10.1038/nature10361
- ↑ Chung KY, Rasmussen SG, Liu T, Li S, DeVree BT, Chae PS, Calinski D, Kobilka BK, Woods VL Jr, Sunahara RK. Conformational changes in the G protein Gs induced by the beta2 adrenergic receptor. Nature. 2011 Sep 28;477(7366):611-5. doi: 10.1038/nature10488. PMID:21956331 doi:10.1038/nature10488
- ↑ Schwartz TW, Sakmar TP. Structural biology: snapshot of a signalling complex. Nature. 2011 Sep 28;477(7366):540-1. doi: 10.1038/477540a. PMID:21956322 doi:10.1038/477540a
See Also
- Nobel Prizes for 3D Molecular Structure
- Highest impact structures of all time
- G proteins
- Rhodopsin
- GTP-binding protein
- Pharmaceutical Drugs
- Membrane proteins
- Hormone
- Ligand Binding N-Terminal of Metabotropic Glutamate Receptors
- Receptor.
Additional Literature
- Carpenter B, Tate CG. Active state structures of G protein-coupled receptors highlight the similarities and differences in the G protein and arrestin coupling interfaces. Curr Opin Struct Biol. 2017 May 5;45:124-132. doi: 10.1016/j.sbi.2017.04.010. PMID:28482214 doi:http://dx.doi.org/10.1016/j.sbi.2017.04.010
External Resources
- Robert J. Lefkowitz and Brian K. Kobilka share the 2012 Nobel Prize in Chemistry for work on GPCRs that includes solving the first structures of a ligand-activated GPCR (2007) and the first activated GPCR in complex with its G protein (2011). A detailed description of the laureates' body of work on this class of receptors with images is here.
- The April 2008 RCSB PDB Molecule of the Month feature on Adrenergic Receptors by David S. Goodsell is 10.2210/rcsb_pdb/mom_2008_4.
- GPCRDB: database contains sequences, ligand binding constants and mutations, in addition GPCR multiple sequence alignments and homology models. Moreover, the site contains useful files where lysozyme and other inserts which are commonly used in the difficult process of crystallizing these transmembrane structures have been removed from the structures.
- GPCR Network site with tracking chart of ongoing structural programs
- The GPCR-SSFE Database: A Homology Model Resource for G-Protein Coupled Receptors
- The blog of the Computational Chemical Biology group at the EMBL-EBI does an excellent job tracking the new GPCR structures as they are emerging.
- Emerald Biosystems Blog that features solved structures and on another page features techniques and amounts needed for crystallization of a number of them.
- A 2012 article from Scripps Research Institute that covers a lot of history of solving the structures of GPCRs and their importance.
- A 2012 article from the Protein Structure Initiative on the screening technique to identify stabilizing fusion partners for solving GPCR structure.
- A 2011 article in Nature entitiled 'Cell Signalling Caught in the Act' describing the first determination of an activated GPCR — the β2 adrenergic receptor (β2AR) — in a complex with its G protein.
- tinyGRAP GPCR mutant database
- GPCR-OKB: GPCR Oligomerization Knowledge Base
- GPCR Natural Variants Database (NaVa)
- The PRED-GPCR server for GPCR recognition and family classification.
- GLASS database: a comprehensive database for experimentally validated GPCR-ligand associations
- GPCR-ModSim is a webserver for computational modeling and simulation of G-Protein Coupled Receptors (GPCRs). Models from sequence and also lets you place the models in a phospholipid bilayer and model them using Molecular Dynamics.
Proteopedia Page Contributors and Editors (what is this?)
Alexander Berchansky, Wayne Decatur, Michal Harel, Ann Taylor, Nikki Hunter